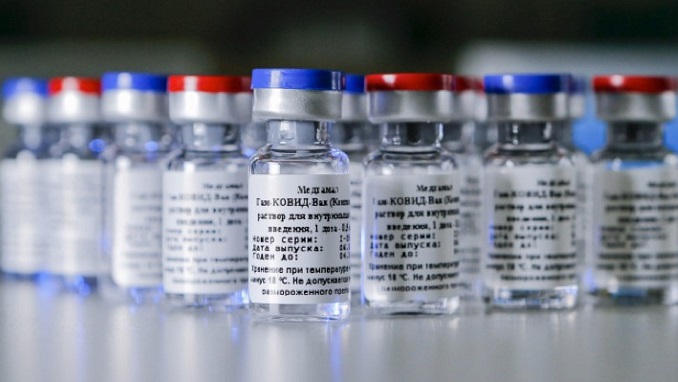
The Russian Direct Investment Fund (RDIF) applied for registration of the Sputnik V vaccine in the European Union on January 29, 2021, and scientific consultations with the European Medicines Agency (EMA) were held on January 19, the fund’s representative told reporters.
“RDIF submitted an application to EMA to participate in a scientific consultation on the Sputnik V vaccine on October 22, 2020. Representatives of the fund, vaccine developers, and EMA representatives held the scientific consultation on January 19. RDIF applied for registration of the Sputnik V vaccine in the European Union on January 29, 2021, and launched the process of submitting information to the EMA through a rolling review procedure,” the fund said.
The fund’s representative also noted that RDIF has an official confirmation from EMA that the application has been accepted, TASS reports. “The rate of approval of the application is determined by the EMA,” the representative said.
The media reported earlier with reference to an EMA representative that the agency had completed consulting the developer of the Russian vaccine Sputnik V, and now the Gamaleya scientific center can apply for registration of the drug in the EU.
Earlier, The Lancet published the results of the Phase Three clinical trials of Russia’s Sputnik V coronavirus vaccine according to which it is one of the safest and most effective ones worldwide. The efficiency of the vaccine amounted to 91.6%, and among volunteers aged over 60 it came to 91.8%. Antibodies to coronavirus after taking the Sputnik V jab were detected in 98% of volunteers.