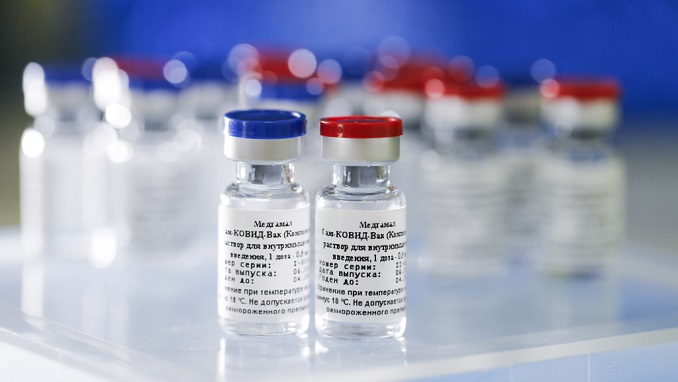
The EU has begun analyzing clinical trial data for Russia’s Sputnik V vaccine — the first step in a process which could lead to the vaccine’s approval for use across the 27-member bloc, The Moscow Times reports.
The European Medicines Agency (EMA) said Thursday it had begun a so-called “rolling review” of Sputnik V, in which it will analyze the existing published data on the vaccine’s safety and effectiveness and decide if there is enough information for Russia to apply for authorization.
Sputnik V was found to have 91.6% efficacy against the coronavirus in Phase 3 trial results published in The Lancet medical journal last month. Russia became the first country in the world to approve a coronavirus vaccine when it authorized the jab for use in August, before any large-scale clinical trials had been conducted.
After international concern over that process, countries have since warmed to the idea of Sputnik V, and it has now been approved for use in more than 40 states.
Announcing the start of the review, the EU said published studies “indicate that Sputnik V triggers the production of antibodies and immune cells … and may help protect against Covid-19.”
Russia has been lobbying the EU — whose vaccination campaign has been beset by delays — to approve its Sputnik V vaccine for use across the bloc. Two members — Hungary and Slovakia — have sidestepped the EMA approval process and already taken shipments of Sputnik V. The president of the Czech Republic has also written directly to President Vladimir Putin requesting a batch of Sputnik V vaccines.
Kirill Dmitriev, CEO of the Russian Direct Investment Fund, which is financing the development and production of Sputnik V, said Russia could provide doses for 50 million Europeans starting in June, should the vaccine be approved.
“We welcome the start of the rolling review procedure by EMA of Sputnik V … Sputnik V can make an important contribution to saving millions of lives in Europe and we are looking forward to a thorough review of data,” Dmitriev said in a statement.
The EU said it was not possible to estimate how long the rolling review would take. In the case of the other vaccines approved for use in the EU — the Pfizer, AstraZeneca and Moderna jabs — it took 2-3 months from the start of the review until the vaccines were granted approval.
The EMA says the process is used to “speed up the assessment of a promising medicine during a public health emergency,” as normally the EMA would not begin looking at any data until a full application has been submitted.
“EMA will assess Sputnik V’s compliance with the usual EU standards for effectiveness, safety and quality,” the regulator said in a statement. “Once the EMA’s human medicines committee decides that sufficient data are available, the company can submit a format application.”
As it would be the first non-Western developed vaccine deployed in the EU, officials have said that Sputnik production sites outside the bloc would need to be inspected.
“They are not producing in Europe, so of course there should be an inspection process on the production sites,” European Commission chief Ursula von der Leyen said on February 17.
Brussels has been wary of Russian and Chinese vaccines, concerned that Moscow and Beijing would use them as soft power tools.
Von der Leyen herself raised questions about why Moscow was so keen to push the vaccine on the EU.
“Overall I must say, we still wonder why Russia is offering theoretically millions and millions of doses while not sufficiently progressing in vaccinating their own people,” she said.