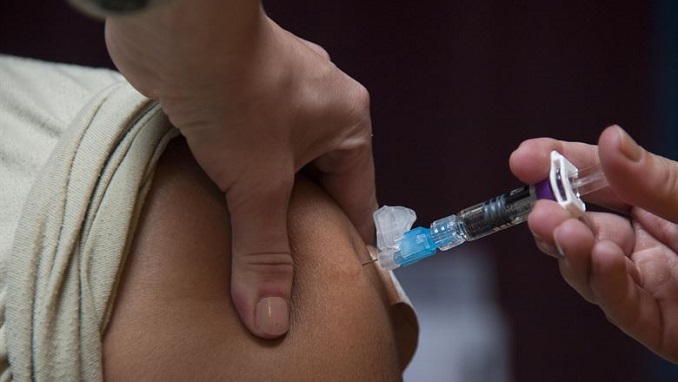
Russia has launched Phase 3 trials of its highly touted coronavirus vaccine to determine the jab’s long-term safety and effectiveness, authorities said Wednesday as questions surrounded the results of its Phase 1 and 2 trials, The Moscow Times reported.
Health Minister Mikhail Murashko said 31,000 out of the announced 40,000 volunteers have been recruited for the study, the state-run TASS news agency reported. Deputy Moscow Mayor Anastasia Rakova said that more than 35,000 Muscovites applied to take part in the trials, according to Interfax.
Neither official specified how many people have taken the adenovirus-based viral vector vaccine so far Wednesday.
Rakova added that the volunteers will receive a second shot of the same vaccine within 21 days of the first.
Russia began first deliveries of the Sputnik V vaccine for the general public this week.
A number of Russian state firm executives, cabinet members, city officials and party leaders have announced in recent weeks that they had received the vaccine.
The announcement comes after a group of prominent scientists highlighted “strange patterns” in the data published in the scientific journal The Lancet last Friday. The data included duplicate values for different groups of patients who had been tested with different formulations of the vaccine, the scientists told The Moscow Times.
The head of the state-run Gamaleya institute that developed the vaccine has also disclosed plans to develop a new vaccine that would double one’s protection from both Covid-19 and the flu.