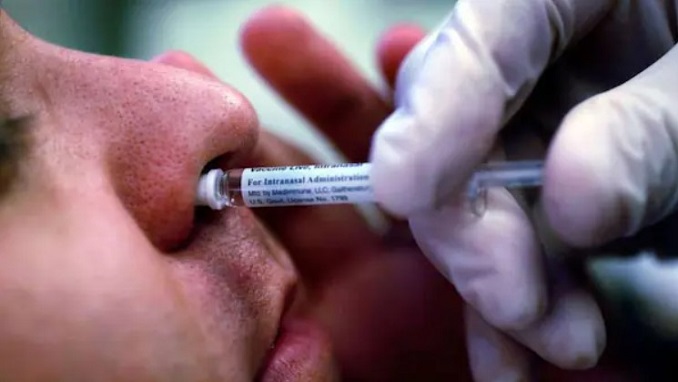
Due to a lack of funding, the anti-coronavirus vaccine in the form of a nasal spray produced by Russia’s Gamaleya Center has yet to reach stage two of clinical testing, according to the center’s chief Alexander Gintsburg.
The studies have been approved, but they have yet to begin, according to Gintsburg, who cited a lack of funding as the cause, TASS reports.
According to information in the relevant registration, the Russian Health Ministry gave permission to the Gamaleya Center on October 13 to undertake Phase Two clinical trials of a coronavirus vaccine in the nasal spray form.
The Gam-COVID-Vac (Sputnik V) preparation will be evaluated with 500 adult volunteers at the Eco-Safety scientific research facility in St. Petersburg, according to the announcement. According to the register, the permission was granted on October 12 and is valid until December 31, 2023.
Alexander Gintsburg, Director of the Gamaleya National Research Center, told TASS at the end of August that trials of the nasal vaccination, which contains Sputnik V’s second component, may begin in late 2021 or early 2022, with certification of the spray likely in 2022.